Abstract
Introduction: Management of patients with hemophilia A is associated with significant economic burden, particularly in high-income developed countries such as Canada, where prophylaxis with replacement FVIII therapies is administered routinely. As standard prophylaxis regimens may lead to over/under treatment in certain individuals, personalized dosing regimens may be more effective for reducing costs.
Aim: This analysis assesses the budgetary impact of a new-generation recombinant FVIII from a human cell line (Nuwiq, simoctocog alfa) for the treatment of patients with hemophilia A in Canada.
Methods: To understand the potential financial impact of introducing simoctocog alfa into the drug reimbursement system of Canada, a budget impact analysis was performed in accordance with the guidelines of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR). A budget impact model was developed from the perspective of the Canadian Health Services considering a five-year time horizon (2017-2021). The model incorporates epidemiological, clinical, market share, cost and resource use data derived from Canadian-specific sources available at the time of model development. In addition to Nuwiq, the FVIII replacement treatments considered in the analysis are antihemophilic factors (recombinant) (Advate, Kovaltry and Eloctate) or antihemophilic factor (Xyntha). Scenario analyses (standard or personalized prophylaxis vs. on-demand) various severity and age groups) were conducted to explore the impact of changing the analysis assumptions. All costs were inflated to 2017 Canadian dollars (CAD).
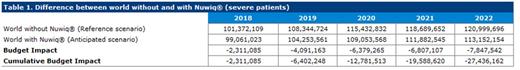
Results : Based on projected market shares over the 5-year time horizon, the introduction of simoctocog alfa to the Canadian market was associated with cost savings for the healthcare system over this time horizon. The cumulative budget impact of gradually replacing existing therapies by Nuwiq® in untreated and previously treated patients with severe hemophilia A over five years was estimated to be -$27,436,162 CAD (cost-saving) (Table 1). The majority of the cost savings (89%, $24,475,207 CAD) are attributed to the use of personalized prophylaxis in patients with severe hemophilia A. When patients with mild and moderate disease were added to the analysis, estimated total savings over 5 years increase further to $31,740,254 CAD. Cost reductions were mainly driven by decreases in the costs of treatment of inhibitor development and bleeding episodes.
Conclusion: Nuwiq® for the treatment of patients with hemophilia A, could be associated with cost savings to the Canadian healthcare system over a time horizon of five years.
Adapa: Octapharma: Employment. Ovcinnikova: Octapharma: Consultancy, Research Funding; Mapi: Employment. Leighton: nspm ltd: Employment; Octapharma: Consultancy, Research Funding. de Silva: Mapi: Employment; Octapharma: Consultancy, Research Funding. Bending: Mapi: Employment; Octapharma: Consultancy, Research Funding. Belyanskaya: Octapharma AG: Employment. Lidia: Octapharma Canada Inc: Employment. Myerson: Octapharma Canada Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal